
- Description
- Oechsle scale
- Thermo-Oechsle scale with automatic temperature correction for reading- Made in Germany
- Alcoholometer
- Thermo-alcoholometer with automatic temperature correction for reading - Made in Germany
- Distillation tail alcoholometer
Alcoholometer and Oechsle scales
Alcoholometer
Basics: Alcoholometers serve by their designation to determine the alcohol content. The exact determination is possible only in a pure alcohol and water mixture. Alcoholometers can measure alcohol because of its different density from water. Here the alcoholometer resembles the Oechsle balance. Both devices measure the density of a solution. What applies to the functioning of the Oechslewaage, the same applies to the alcoholometer. The alcoholometer also measures the density of a solution compared to water. Alcohol has a lower density than water. The more alcohol in a solution, the deeper into this solution will therefore immerse the alcoholometer. However, if substances are simultaneously present in the solution that increase the density (for example, sugar), then the alcoholometer can not submerge accordingly to the alcohol content. The measurement is too low. In a fermentation, e.g. an alcohol meter can not measure the alcohol content.
Instructions: The liquid to be measured is stirred well and then filled into a spindle cylinder of appropriate size. The purified alcoholometer is then carefully lowered into the liquid. If the alcoholometer has reached the equilibrium position and floats freely, it is read. The higher the alcohol content, the deeper it dips into the liquid. Immediately after reading, the temperature of the liquid is checked. If it deviates from the reference temperature of 20 ºC, the reading should be corrected as follows, depending on the reading accuracy required: per degree above 20 ºC, 0.2% vol. counted per degree below 20 ° C are 0.2% vol. added counted. You can also apply the correction using the following script:
Temperature correction of the alcohol display in Vol .-%
Oechsle scale
Basics: The Oechslegrad indicates how much heavier or lighter one liter of the measured liquid is than one liter of pure water.
Two examples:
· 1 liter of a liquid weighs 1050 g. This liquid therefore contains 1050 - 1000 = 50 degrees Oechsle.
· 1 liter of another fluid weighs 975 g. This fluid contains 975 - 1000 = -25 degrees Oechsle.
The more oechslegrade, the sweeter the liquid. Since the density of a liquid depends on its temperature and the Oechsle scales are calibrated to a temperature of 20 ° C, the reading must be corrected if the temperature of the liquid differs significantly from 20 ° C. 0.2 ° C Oechsle is added per ° C above 20 ° C, 0.2 ° C Oechsle must be withdrawn per ° C below 20 ° C.
Instructions: To determine the Oechsle degrees fill a measuring cylinder with the liquid to be measured and then let the Oechslewaage slowly slide into it until it floats free. The balance must be vertical in the liquid and must not touch the wall. It also needs to be clean and there must be no air bubbles on it. If necessary, the carbon dioxide in the already fermenting mash must be removed by vigorous shaking. The reading is made on the scale at the level of the liquid level. Aqueous solutions form a so-called "meniscus", i. the liquid pulls up on the glass. The reading can now be "up" or "down". Normally the Oechsle scale shows which model is the right type for your model. If there is no indication, the reading is always "down". One receives an indication in "Oechsle degrees".
Oechsle scale and mash alcohol indicator with plastic hard box
260 mm total length
0 - 120° degree Oechsle in the must measuring range
0 - 20 Vol .-% in the mash alcohol measuring range (only with complete fermentation)
With the Oechslesscale the density is measured in so-called Oechsle levels. Traditionally, the density of must (wine approach) is referred to as must weight, the Oechsles-cale is therefore sometimes called Mostwaage.
Instructions: To determine the Oechslegrade fill a measuring cylinder with the liquid to be measured and then let the Oechslewaage slowly slide into it until it floats free. The balance must be vertical in the liquid and must not touch the wall. It also needs to be clean and there must be no air bubbles on it. If necessary, the carbon dioxide in the already fermenting mash must be removed by vigorous shaking. The reading is made on the scale at the level of the liquid level. Aqueous solutions form a so-called "meniscus", i. the liquid pulls up on the glass. The reading can now be "up" or "down". Normally the Oechsle-scale shows which model is the right type for your model. If there is no indication, the reading is always "down". One receives an indication in "degree of Oechsle".
Basics: The degree Oechsle indicates how much heavier or lighter one liter of the measured liquid is than one liter of pure water.
Two examples:
· 1 liter of a liquid weighs 1050 g. This liquid therefore contains 1050 - 1000 = 50 degrees Oechsle.
· 1 liter of another fluid weighs 975 g. This fluid contains 975 - 1000 = -25 degrees Oechsle.
The more oechslegrade, the sweeter the liquid. Since the density of a liquid depends on its temperature and the Oechsle scales are calibrated to a temperature of 20 ° C, the reading must be corrected if the temperature of the liquid differs significantly from 20 ° C. 0.2 ° C Oechsle is added per ° C above 20 ° C, 0.2 ° C Oechsle must be withdrawn per ° C below 20 ° C.
Thermo-Oechsle scale with automatic temperature correction for reading
Sugar content in °Oechsle
with plastic hard box
333 mm total length
0 - 130 º Oechsle
Temperature range 0 ºC to 35 ºC
Oechsle's must scales are used to determine the specific gravity of sugar solutions made from sweet grapes, fruit juices, fruit juices and liquid mash. Oechsle grades indicate by how many grams 1 liter of the sugar-containing solution is heavier than water at a reference temperature of 20 ° C, which is the temperature at which the device was calibrated. If the temperature is higher, the liquid expands and a liter is lighter, at lower temperature it contracts and one liter is heavier. Therefore, the measuring scale then displays too low or too high a value. This so-called measurement error is eliminated with the help of the correction scale.
Manual:
To determine the Oechslegrade fill a 250 ml measuring cylinder high mold with the liquid to be measured and then let the Oechsle scale slowly slide into it until it floats free. The balance must be vertical in the liquid and must not touch the wall. It also needs to be clean and there must be no air bubbles on it. If necessary, the carbon dioxide in the already fermenting mash must be removed by vigorous shaking. The reading is made on the scale at the level of the liquid level. The higher the sugar content (must weight), the higher it rises in the liquid. The temperature is recorded simultaneously with the built-in thermometer. The picked must weight in Oechsle or Brix is exactly at the calibration temperature of 20 ºC. If the temperature is higher, too low a value is displayed, it is lower, too high a value. This measurement error can be read directly next to the temperature display below. The displayed correction value must now only be counted or subtracted from the read Oechsle or Brix value to obtain the correct value. Aqueous solutions form a so-called "meniscus", i. the liquid pulls up on the glass. The reading can now be "up" or "down". Normally the Oechslewaage shows which model is the right type for your model. If there is no indication, the reading is always "down". One gets an indication in "degree Oechsle" or Brix.
Basics:
The degree Oechsle indicates how much heavier or lighter one liter of the measured liquid is than one liter of pure water.
Two examples:
· 1 liter of a liquid weighs 1050 g. This liquid therefore contains 1050 - 1000 = 50 degrees Oechsle.
· 1 liter of another fluid weighs 975 g. This fluid contains 975 - 1000 = -25 degrees Oechsle.
The more oechslegrade, the sweeter the liquid. Since the density of a liquid depends on its temperature and the Oechsle scales are calibrated to a temperature of 20 ° C, the reading must be corrected if the temperature of the liquid differs significantly from 20 ° C. 0.2 ° C Oechsle is added per ° C above 20 ° C, 0.2 ° C Oechsle must be withdrawn per ° C below 20 ° C. The done with the present device, the correction scale.
Alcoholometer with plastic hard box
257 mm total length
0-100 vol.% In an alcohol-water mixture

Instructions: The liquid to be measured is stirred well and then filled into a spindle cylinder of appropriate size. The purified alcoholometer is then carefully lowered into the liquid. If the alcoholometer has reached the equilibrium position and floats freely, it is read. The higher the alcohol content, the deeper it dips into the liquid. Immediately after reading, the temperature of the liquid is checked. If it deviates from the reference temperature of 20 ºC, the reading should be corrected as follows, depending on the reading accuracy required: per degree above 20 ºC, 0.2% vol. counted per degree below 20 ° C are 0.2% vol. added counted. You can also apply the correction using the following script:
Temperature correction of the alcohol display in Vol .-%
Basics: Alcoholometers serve by their designation to determine the alcohol content. The exact determination is possible only in a pure alcohol and water mixture. Alcoholometers can measure alcohol because of its different density from water. Here the alcoholometer resembles the Oechsle balance. Both devices measure the density of a solution. What applies to the functioning of the Oechslewaage, the same applies to the alcoholometer. The alcoholometer also measures the density of a solution compared to water. Alcohol has a lower density than water. The more alcohol in a solution, the deeper into this solution will therefore immerse the alcoholometer. However, if substances are simultaneously present in the solution that increase the density (for example, sugar), then the alcoholometer can not submerge accordingly to the alcohol content. The measurement is too low. In a fermentation, e.g. an alcohol meter can not measure the alcohol content.
Thermo-alcoholometer with automatic temperature correction for reading
with plastic hard box
290 mm total length
0-100 vol.% In an alcohol-water mixture
Temperature range 0 ºC to 35 ºC

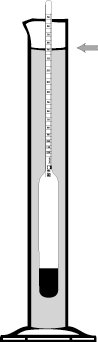
Instructions: The liquid to be measured is stirred well and then filled into a spindle cylinder of appropriate size. The purified alcoholometer is then carefully lowered into the liquid. If the alcoholometer has reached the equilibrium position and floats freely, it is read. The higher the alcohol content, the deeper it dips into the liquid. The temperature is recorded simultaneously with the built-in thermometer. The read alcohol% by volume value applies exactly at the gauge temperature of 20 ° C. If the temperature is higher, too high a value is displayed, it is lower, too low a value. This measurement error can be read directly next to the temperature display below. The displayed correction value now only has to be deducted or counted from the read vol.% Value in order to obtain the correct value.
Basics: Alcoholometers serve by their designation to determine the alcohol content. The exact determination is possible only in a pure alcohol and water mixture. Alcoholometers can measure alcohol because of its different density from water. Here the alcoholometer resembles the Oechsle balance. Both devices measure the density of a solution. What applies to the functioning of the Oechslewaage, the same applies to the alcoholometer. The alcoholometer also measures the density of a solution compared to water. Alcohol has a lower density than water. The more alcohol in a solution, the deeper into this solution will therefore immerse the alcoholometer. However, if substances are simultaneously present in the solution that increase the density (for example, sugar), then the alcoholometer can not submerge accordingly to the alcohol content. The measurement is too low. In a fermentation, e.g. an alcohol meter can not measure the alcohol content.Distillation tail alcoholometer with plastic hardbox
170 mm total length,
especially used for the measurement of smaller samples in the destillation tail
0-100 vol.% In an alcohol-water mixture

Instructions: The liquid to be measured is stirred well and then filled into a spindle cylinder of appropriate size. The purified alcoholometer is then carefully lowered into the liquid. If the alcoholometer has reached the equilibrium position and floats freely, it is read. The higher the alcohol content, the deeper it dips into the liquid. Immediately after reading, the temperature of the liquid is checked. If it deviates from the reference temperature of 20 ºC, the reading should be corrected as follows, depending on the reading accuracy required: per degree above 20 ºC, 0.2% vol. counted per degree below 20 ° C are 0.2% vol. added counted. You can also apply the correction using the following script:
Temperature correction of the alcohol display in Vol .-%
Basics: Alcoholometers serve by their designation to determine the alcohol content. The exact determination is possible only in a pure alcohol and water mixture. Alcoholometers can measure alcohol because of its different density from water. Here the alcoholometer resembles the Oechsle balance. Both devices measure the density of a solution. What applies to the functioning of the Oechslewaage, the same applies to the alcoholometer. The alcoholometer also measures the density of a solution compared to water. Alcohol has a lower density than water. The more alcohol in a solution, the deeper into this solution will therefore immerse the alcoholometer. However, if substances are simultaneously present in the solution that increase the density (for example, sugar), then the alcoholometer can not submerge accordingly to the alcohol content. The measurement is too low. In a fermentation, e.g. an alcohol meter can not measure the alcohol content.
